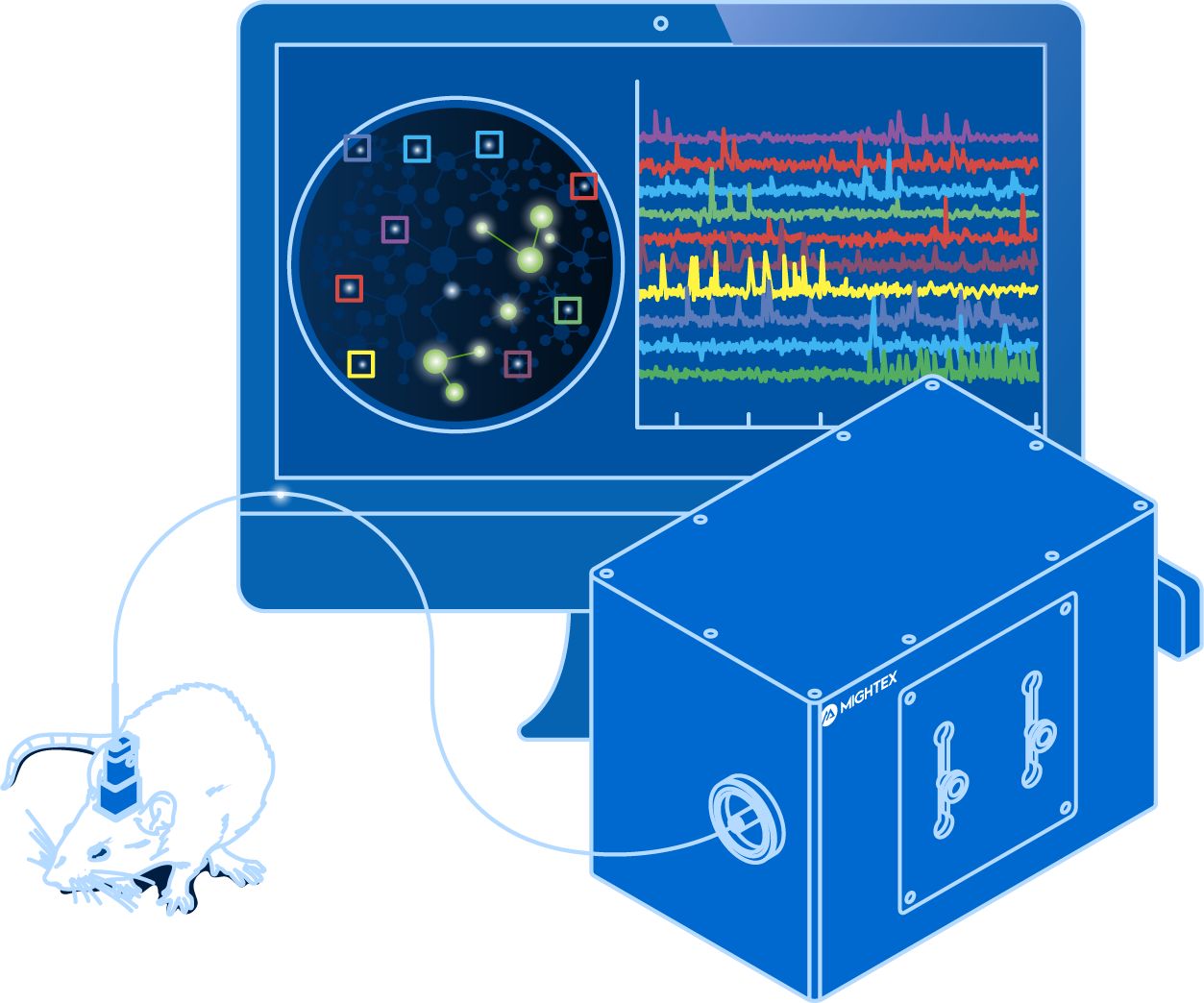
The invention of optogenetics has enabled scientists to use light to turn cell-type specific neural activity on or off with millisecond-precision to probe neural circuit function. Complementing optogenetics, calcium imaging has provided scientists with a method to observe cell-type specific neural circuit activity with cellular-resolution using fluorescent indicators.
The integration of optogenetics and calcium imaging opens the door to these techniques being performed simultaneously in the same experiment to investigate the causal link between neural circuit activity, function, and behaviour. All-optical optogenetics and calcium imaging can enable scientists to image the activity of neurons in vivo and simultaneously perturb cellular activity using optogenetics in the same animal to both ‘read’ and ‘write’ neural activity.
To perform all-optical experiments with both calcium imaging and optogenetics, scientists require both optimized biology and advanced optical systems.
All-Optical Biology
Optogenetic probes have been developed with different functions (excitation or inhibition), activation times, and expression properties.
They are excited by wavelengths of light ranging from blue to red, depending on their properties. Channelrhodopsin (ChR2) has been the optogenetic tool of choice for excitation of neural activity and halorhodopsin for inhibition due to their extensive development and use in the field of optogenetics. Optogenetic probes have only an excitation spectrum to activate their excitation/inhibitory properties (Yizhar et al. 2011).
In contrast, GECIs behave in the same manner as a fluorophore (e.g., GFP), such that they exhibit excitation and emission spectra. However, the fluorescent signal of GECIs is dependent on intracellular calcium concentrations (i.e., increased fluorescence emission due to increased calcium influx) and displays dynamic behaviour, unlike a static signal emitted from a fluorophore (Grienberger & Konnerth 2012).
Researchers commonly propose the use of GCaMP for imaging and channelrhodopsin (ChR2) for optogenetics in all-optical experiments due to the optimization, efficiency, and frequent use of these biological probes.
However, a problem occurs with this combination: GCaMP and ChR2 have overlapping excitation spectrums (peak wavelength ~470nm). When GCaMP and ChR2 are expressed in the same tissue, calcium imaging light excitation can potentially activate the optogenetic probe as well. Thus, GCaMP imaging and ChR2 optogenetic stimulation cannot be performed simultaneously due to potential optical crosstalk. It is not possible to determine if measured changes in GCaMP signals are due to natural changes in neural activity or optogenetic-induced changes.
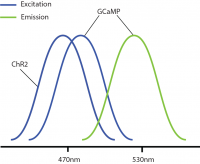
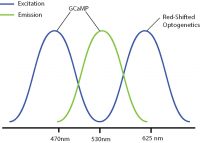
Optical crosstalk in all-optical experiments can be reduced by selecting imaging indicators and optogenetic probes with non-overlapping excitation spectrums (Emiliani et al. 2015).
Examples of combinations with reduced optical crosstalk are blue excitation/green emission imaging (e.g. GCaMP) and red-shifted optogenetics (e.g. Chrimson, Jaws) or green excitation/red emission imaging (e.g. RCaMP) and blue-shifted optogenetics (e.g. ChR2, GtAChR). Although there may be some overlap between the excitation spectrums of these probes, the likelihood of crosstalk will be reduced, preventing indirect activation of your optogenetic construct during imaging.
The further optimization of imaging and optogenetics probes (e.g. sensitivity, excitation spectra) will help prevent optical crosstalk in all-optical optogenetics and calcium imaging experiments.
All-Optical Systems
1. Fiber Photometry
Optogenetics can be integrated into fiber photometry experiments. The only additional equipment required is the appropriate LED for optogenetic stimulation and filter set to combine with the calcium imaging LED. An example includes adding a red LED for red-shift optogenetics and GCaMP imaging (Kim et al. 2016).
Similar to calcium imaging with fiber photometry, optogenetic stimulation can only be performed widefield, stimulating all neurons with no cellular resolution.
A key feature of fiber photometry is the possibility to perform multi-region optogenetics in combination with multi-region calcium imaging; however, in this setup, due to technical limitations, optogenetic stimulation cannot be conducted in one select region at a time, and instead, it illuminates all regions simultaneously (Kim et al. 2016).
Fiber photometry is a simplistic method to perform population all-optical calcium imaging and optogenetics experiments in one or multiple brain regions.
2. Miniscope
Recently, miniscopes have integrated optogenetics (Stamatakis et al. 2018). This requires an additional LED and the appropriate filter set to collect the correct signal. Unlike fiber photometry, the LED and the filter are directly integrated into the miniscope (2~3g in weight), and this creates additional weight on the head of the animal.
Due to the inability of integrating a DMD pattern illuminator or a laser scanner, miniscopes are restricted to performing widefield optogenetics, with no cellular resolution. Miniscopes can be used to visualize individual neurons with cellular resolution, but they can only optogenetically stimulate all cells.
Also, miniscopes are limited to performing optogenetics and imaging in a single brain region due to the relatively bulky size and heavyweight of the system.
3. Optical Fiberscope
Compared to the optical systems described above, an optical fiberscope (such as Mightex’s OASIS Implant system) is an extremely flexible system for all-optical calcium imaging and optogenetics. This system can easily be upgraded with optogenetics capabilities by simply adding the appropriate LED and filter set combination.
In comparison to both the miniscope and fiber photometry, the optical fiberscope can not only perform widefield optogenetics but also, by integrating a digital-mirror-device (DMD), be used to perform cellular-resolution optogenetics in vivo. The optical fiberscope is the only freely-behaving system capable of cellular-resolution calcium imaging and cellular-resolution optogenetics in a freely-behaving animal. Scientists can perform these experiments in the deep-brain or across a large area of the cortex.
For larger field of view or higher-power applications, lasers (instead of LEDs) can be used for optogenetics using the optical fiberscope
Researchers can perform cellular-resolution calcium imaging and optogenetics in multiple regions simultaneously by using a split-fiber with the optical fiberscope. For example, one can stimulate multiple individual neurons or a selected group of neurons in one region and image individual cells in another, and this is not possible with fiber photometry or a miniscope.
The optical fiberscope is a powerful all-optical imaging and optogenetics system capable of both cellular-resolution optogenetics and cellular-resolution imaging in a single brain region or multiple brain regions simultaneously.
System Comparison Table