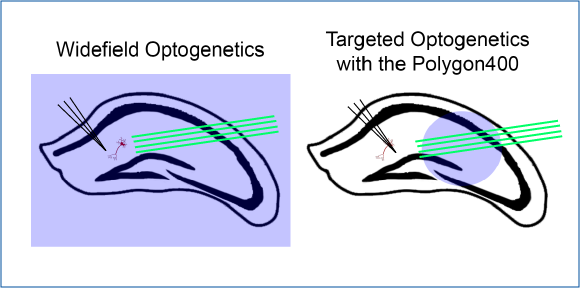
Optogenetics has advanced the field of neuroscience by providing a precise method for manipulating neuronal activity. The ability to perform cellular-resolution optogenetics enables researchers to stimulate select optogenetic-expressing neurons (e.g. ChR2, NpHr, Chrimson etc.), individually or simultaneously, in slice or culture during electrophysiological recordings or calcium imaging to study the correlation between neural activity and function, or to map neural circuitry. Below you can find application examples.
Circuit Mapping with Cellular-Resolution Optogenetics
Andrasi et al. 2017 investigated the connection between principal neurons and basket cells in the amygdala. Whole-cell slice electrophysiology was used to measure amygdala activity, and ChR2 was expressed to manipulate network activity.
Mightex’s Polygon was used to illuminate individual principal neurons expressing ChR2 and the activity elicited from a basket neuron was measured.
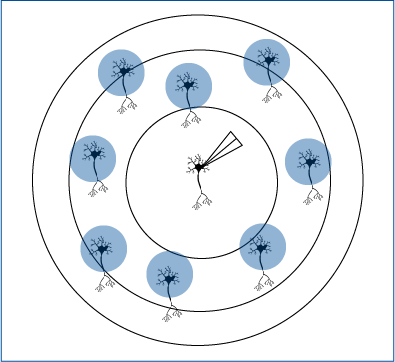
Zebrafish Responses Controlled by Targeted Optogenetics
Tabor et al. 2018 investigated in zebrafish the involvement of a subset of neurons in sensory filtering. The zebrafish’s behavioural response was measured in response to ChEF optogenetic stimulation.
Mightex’s Polygon was used to illuminate all neurons expressing ChEF or a subset of these neurons and the behaviour elicited was examined.
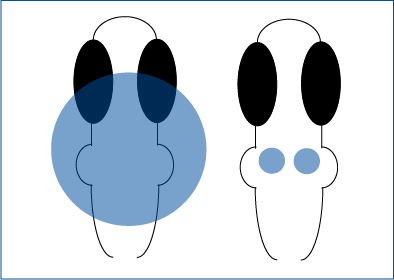
Optogenetic Stimulation of Axons-Only
Takacs et al. 2018 explored the characteristics of basal forebrain projections to the hippocampus Whole-cell slice electrophysiology was used to measure hippocampal activity, and ChR2 was expressed in projecting neurons from the basal forebrain.
Mightex’s Polygon was used to illuminate individual projecting axons from the basal forebrain, excluding terminals, to explore the effects of these manipulations.
Grid-Scanning Optogenetics to Explore Neural Circuits
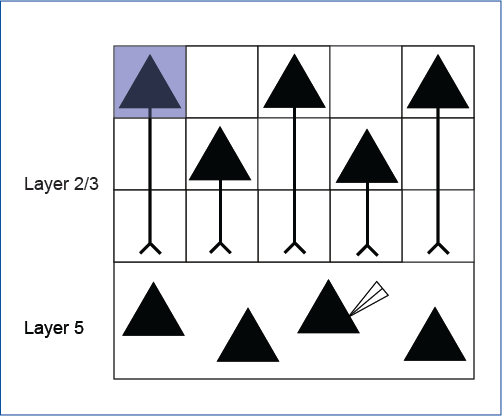
Simonova et al. 2019 explored heterosynaptic plasticity in the neocortex. Whole-cell slice electrophysiology was used to record from layer 5 neurons, and ChR2 was expressed in layer 2/3 neurons to manipulate layer 5 activity.
Mightex’s Polygon was used to illuminate a randomized grid pattern across layer 2/3 neurons and the activity elicited from a layer 5 was measured.
Layer-Specific Optogenetics in the Hippocampus
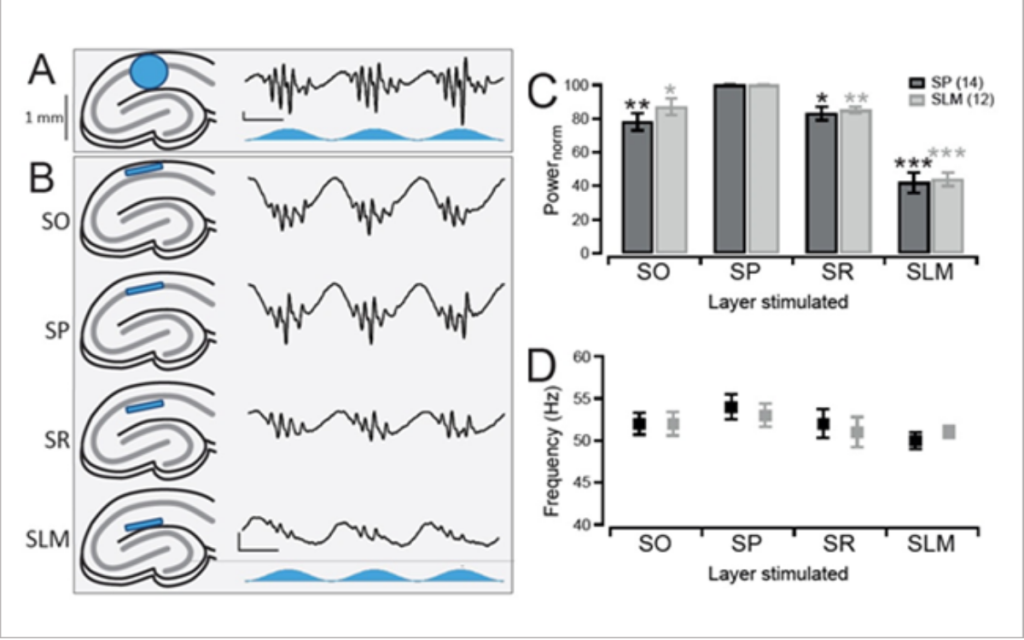
Butler et al. 2016 explored intrinsic production of gamma oscillations in CA1 region of the hippocampus. Slice electrophysiology was used to measure hippocampal activity, and ChR2 was expressed to manipulate network activity.
Mightex’s Polygon was used to illuminate select layers of the hippocampus expressing ChR2 to measure the oscillatory activity produced.
Resources
Cellular-Resolution Optogenetics
with Mightex’s Polygon
Contact Us About Polygon
"*" indicates required fields