Intrinsic optical imaging has its strengths and weaknesses, like many techniques. Many researchers employ this technique because it is non-invasive, requiring very little surgical intervention and minimal equipment (Juavientt et al., 2018). Intrinsic optical imaging provides a large field-of-view with relatively good spatial and temporal precision for individuals studying activity across the whole-cortex or large cortical regions (Lu et al., 2017).
However, intrinsic optical imaging lacks in certain areas. First, this technique provides measurement of global neural activity with no ability to target specific neuron populations (e.g. excitatory vs inhibitory). Second, depending on what is being studied, intrinsic imaging may have too slow temporal resolution, with seconds precision, or too low of spatial resolution, with no ability to visualize individual neurons (Juavientt et al., 2018). Lastly, intrinsic optical imaging is solely used for recording neural activity and cannot be used for manipulating neural activity to test potential causal relationships.
The limitations of intrinsic optical imaging do not necessarily require replacing the technique; instead, these limitations can be addressed by using complementary optical techniques. The integration of techniques such as fluorescent activity indicators and optogenetics can greatly benefit intrinsic optical imaging experiments.
Fluorescent Activity Indicators
Fluorescent activity indicators, such as genetically-encoded calcium and voltage indicators, can help overcome the spatial and temporal limitations of intrinsic imaging. These indicators are genetically expressed and identified through fluorescence emission (Grienberger & Konnerth, 2012), in comparison to intrinsic optical imaging that simply requires reflected light (Hillman et al., 2008). The genetic expression of these indicators makes them capable of identifying explicit cellular population activity.
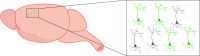
In addition, changes in fluorescence of activity indicators allows for identification of individual cells (Grienberger & Konnerth, 2012), unlike intrinsic imaging that only detects global activity. Fluorescent activity indicators also display quicker temporal dynamics (Lin & Schnitzer, 2017), which may be important depending on the experiment.
Since both techniques require light to function, they are complementary to each other and can be integrated into the same optical system. There are two potential setups for integrating fluorescent imaging indicators with optical intrinsic imaging.
First, a standard mesoscope setup can be used with both techniques. This allows for large field-of-view imaging. Intrinsic imaging may help identify regions, while fluorescent imaging indicators provide higher temporal dynamics and specific cell-type population activity changes across the cortex.
Second, one can potentially use large-scale intrinsic optical imaging to detect regional changes with a mesoscope, and combine this with high-resolution two-photon imaging (Morone et al. 2017). This combination provides the ability to not only detect large-scale changes, but also activity changes at the single-cell level to correlate global and single-cell activity.
The downside to using fluorescent activity indicators is they require optimization of expression, more intensive surgical procedures, and additional equipment. Compared to intrinsic imaging that uses the intrinsic properties of the brain, fluorescent activity indicators must be expressed in the brain of the animal (Resendez et al., 2016). This necessitates intense optimization of viral expression to achieve high imaging-quality data.
Furthermore, fluorescent indicators require surgical intervention to implant a cortical window and express the virus, making it a more invasive imaging technique (Resendez et al., 2016). Lastly, the addition of the appropriate filter set and excitation light source is necessary to both excite the indicator and image its fluorescent emission.
In summary, fluorescent activity indicators can complement intrinsic imaging experiments by providing higher temporal precision, cell-type specificity, and spatial precision while recording neural activity across the cortex.
Optogenetics
While intrinsic optical imaging provides large-scale measurement of neural activity across the cortex, this technique is unable to manipulate neural activity, which is essential for scientists to study causal relationships between cortical connections, behaviour, and sensory responses.
The introduction of optogenetics, however, has provided scientists with an optical technique to perturb neural activity in a well-controlled manner. By using light-activated channels that can be genetically expressed in the brain, optogenetics can be used to manipulate neural activity with high temporal precision and cell-type specificity (Boyden et al., 2005).
With both intrinsic optical imaging and optogenetics using light, these techniques can be integrated and complement each other to provide cortical imaging and manipulation (Nakamichi et al., 2019). Two methods of optogenetics can be performed with intrinsic imaging across the cortex using a mesoscope: widefield and targeted.
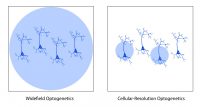
Widefield optogenetics can be used to perturb activity across the entire field of view with no spatial precision. In comparison, targeted optogenetics, based on (for example) Mightex’s Polygon DMD Illuminator, enables scientists to target individual parts of the cortex (or defined groups of neurons), in order to study how cortical areas or certain neuron populations influence cortical activity.
Although optogenetics is used to manipulate neural activity, it requires genetic expression and surgical invasiveness. Similar to calcium imaging, expression of the optogenetic probe may need to be optimized to achieve peak activation or inhibition of neural activity (Mei & Zhang, 2012). In addition, the implantation of a cortical window may be required to perform optogenetics, increasing the invasiveness of the technique.
The other consideration of using optogenetics with intrinsic imaging is overlapping excitation wavelengths. Depending on the experiment, both techniques may use the same excitation wavelengths; thus, intrinsic imaging may induce optogenetic activation or inhibition leading to unintended activity changes. It is, therefore, crucial to either select an optogenetic probe that has a non-overlapping excitation wavelength with intrinsic imaging or select a non-overlapping wavelength for intrinsic imaging (Emiliani et al., 2015).
Optogenetics can complement intrinsic imaging by providing the ability to manipulate neural activity with high temporal precision, cell-type specificity, and spatial precision to test causal relationships.
Conclusion
Intrinsic optical imaging has greatly contributed to research studying the cortex by providing a non-invasive technique to record neural activity. The development of fluorescent indicators and optogenetics can help advance and complement intrinsic optical imaging experiments by providing cell-type specificity, temporal precision, single-cell precision, and manipulation of neural activity.