Free eBook Download
Intrinsic Optical Imaging: The Ultimate Guide
Intrinsic Optical Imaging: The Ultimate Guide
Intrinsic optical imaging (or intrinsic signal imaging) is an imaging method that enables scientists to indirectly record neural activity non-invasively by measuring hemodynamic changes in the brain
This guide explores questions related to intrinsic optical imaging, including what is intrinsic optical imaging? What equipment is necessary for intrinsic imaging?
Current advanced imaging techniques, such as calcium imaging, can record cortical activity with high spatial precision and cell-type specificity using the expression of fluorescent sensors and implantation of a cortical window; however, this technique can be quite invasive and complex.
For head-fixed experiments measuring general changes in cortical activity in different areas, there is not always a need for such complex imaging techniques, but rather a simplistic imaging method is more suitable to examine basic physiology.
Is there a more simplistic imaging method available?
Intrinsic Optical Imaging
Intrinsic optical imaging (or intrinsic signal imaging) is an imaging method that enables scientists to indirectly record neural activity non-invasively by measuring hemodynamic changes in the brain (Morone et al., 2017).
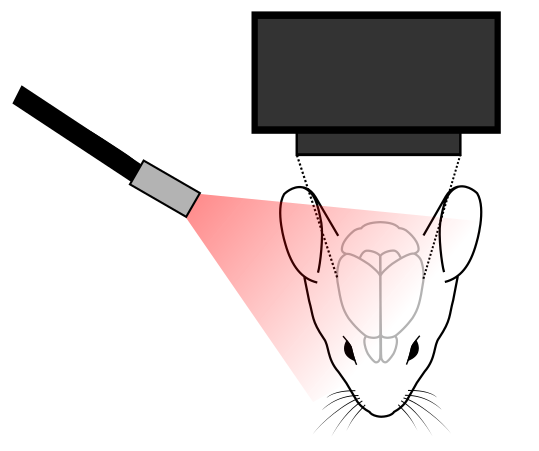
In a typical setup, a mesoscope is used to image the cortex of an animal, and an external light source is used to shine light onto the cortex off which light will then be reflected. The camera on the mesoscope detects slight changes in reflected signal off the cortex, which is indicative of neural activity (Hillman, 2008). These changes are quite small (1% change), and they are measured relative to a baseline control (Morone et al., 2017).
Specifically, intrinsic optical imaging exploits the differential spectral absorption properties of oxygenated and deoxygenated hemoglobin (Hillman, 2008). Where the typical response around the site of neural activity shows an increase in deoxygenated hemoglobin and blood flow followed by an increase in oxygenated hemoglobin (Juavientt et al., 2018). These changes can be detected by using different wavelengths for total hemoglobin and blood flow (500-599nm) and deoxyhemoglobin (600-699nm) measurement (Morone et al., 2017). Thus, most intrinsic imaging experiments use multiple wavelengths to detect different components in the hemodynamic response related to neural activity.
In cortex-wide or cortical imaging experiments, intrinsic optical imaging is commonly used to measure changes in neural activity due to a behaviour or presentation of a stimulus. The relative temporal and spatial precision of intrinsic imaging is normally appropriate for these types of experiments (Juavientt et al., 2018).
A common reason neuroscientists employ intrinsic optical imaging—compared to other more advanced imaging techniques (e.g. calcium imaging)—is to image neural activity in large cortical areas and examine basic physiology (Juavientt et al., 2018). For these applications, intrinsic optical imaging provides both high enough spatial (100um) and temporal resolution (1-2 seconds to peak) to map stimulus-evoked cortical activity in a head-fixed animal (Lu et al., 2017).
One advantage of intrinsic optical imaging is being able to image neural activity with minimal invasiveness and no expression of fluorescent reports. Due to the simplicity of this imaging technique, intrinsic optical imaging does not necessitate the implantation of a cortical window to replace the skull or thinning of the skull (Juavientt et al., 2018); however, if combined with other imaging techniques, such as two-photon imaging, a cortical window would be necessary. Using the reflected properties of hemodynamic changes in the brain provides a convenient way to image neural activity without the need for fluorescent reporters to be expressed (Hillman, 2008). These two factors, in combination with minimal equipment, makes intrinsic imaging a fairly inexpensive tool to probe neural activity across large cortical regions.
With such ease of use, there are potential pitfalls associated with intrinsic imaging, and these are mostly dependent on the application being studied. First, due to the inherent properties of the brain, there is light scattering associated with intrinsic imaging and thus, this method is limited to superficial regions of the cortex (Hillman, 2008). Second, intrinsic optical imaging cannot provide single-cell or cell-type specific imaging. This limitation is due to the spatial resolution of the technique and solely relying on hemodynamic changes, which cannot provide cell-type specific changes (Juavientt et al., 2018). Thus, intrinsic optical imaging is more useful as a general activity reporter across the cortex. Third, depending on the cortical events being studied or behavioural stimuli, intrinsic imaging may be too slow to measure changes in cortical activity (Lu et al., 2017). Intrinsic optical imaging reflects changes over a matter of seconds, and this may not be ideal for certain experiments.
To overcome the limitations of intrinsic optical imaging, it would be appropriate to implement or complement these experiments using more advanced techniques such as calcium imaging, voltage imaging, or voltage sensitive dyes. In particular, calcium imaging is a well-suited and established imaging technique for resolving single-cells or cell-type specific activity and measuring neural activity with higher temporal precision.
Intrinsic optical imaging (or intrinsic signal imaging) is a simplistic imaging method that enables scientists to indirectly record neural activity in large cortical regions non-invasively by measuring hemodynamic changes in the brain (Morone et al., 2017).
You’re probably asking, what are the necessary components to perform intrinsic optical imaging?
An important reason researchers employ intrinsic optical imaging to observe large-scale neural activity is the non-invasive nature of the technique, which is composed of fewer biological components, compared to other techniques, such as calcium imaging.
Intrinsic optical imaging does not require the expression of fluorescent reporters to record neural activity, and hence, sophisticated surgical procedures for viral transfection or generation of transgenic lines are not necessary, unlike calcium imaging. This technique then requires one less surgical procedure or variable to take into account for experiments and, instead, it measures hemodynamic changes in the brain based on slight changes in reflected light off the cortex (Morone et al., 2017).
However, to image activity in the brain, the skull must be exposed. For intrinsic imaging, it is possible to image through the skull. In this case, a window may be glued over the exposed skull, further reducing the invasiveness and simplifying preparation for this technique. This is compared to having to thin the skull or inserting a cortical window for imaging the brain directly.
Some researchers may choose to employ other more invasive techniques in combination with intrinsic optical imaging, such as two-photon imaging which requires the insertion of a cortical window.
Intrinsic imaging is commonly employed for large-scale imaging because of limited biological components and a simple list of equipment: mesoscope, light source, filter, and camera.
Generally, intrinsic optical imaging is employed for large-scale cortical imaging. This may be imaging a single-cortical region (e.g. 4 mm x 4 mm) or whole-cortex (e.g. 10 mm x 10 mm) in mice. With such a large field of view, a standard microscope cannot be used. Rather, a mesoscope, such as Mightex’s OASIS Macro, is used to image large cortical areas, collect the reflected signal, and direct it to the camera. A mesoscope can be used with 0.5x to 4x objectives to image and capture light from the whole cortex of a head-fixed animal. This enables neural activity imaging across multiple cortical brain regions simultaneously.

In order to acquire quality data, a scientific camera is oftentimes required to efficiently collect reflected signal from the brain and to detect minute neural activity changes. For most intrinsic optical imaging applications, researchers will usually use a charge-coupled device (CCD) camera due to the high-sensitivity (Morone et al., 2017), and such a sensitive camera is required because changes in intrinsic optical imaging signals are very small (<1%)(Morone et al., 2017).
In addition, using a high frame-rate camera is important when it comes to faithfully capturing the spatio-temporal optical signal and synchronizing the camera with the light source operating in pulse mode (Morone et al., 2017; Hillman, 2008) Furthermore, a camera with a higher signal-to-noise ratio will enable scientists to detect minute changes in the intrinsic optical imaging signal and gather data which otherwise would have been lost (Juavientt et al., 2018). Lastly, a camera with a large field of view is required for imaging large portions of the cortex, such as whole-cortex imaging (Morone et al, 2017; Juavientt et al., 2018).

As mentioned above, intrinsic optical imaging does not necessarily require fluorescent reports, and hence, there is usually no need to use a standard fluorescence filter set in intrinsic imaging . However, the use of an excitation filter on the light source can help select the desired optical wavelengths, be it from an LED light source or a halogen light source. In addition, individuals performing these experiments may employ a bandpass filter in front of the camera to reduce extraneous light that is not reflected from the brain, and this will help to significantly improve the signal-to-noise ratio (Juavientt et al., 2018).
A light source needs to be used to illuminate the cortex and to produce reflected signals from the cortex to the camera. Thus, it is crucial to have good lighting to acquire good signal for intrinsic optical imaging (Sobottoka et al., 2013).
There are a wide-range of light sources that can be employed for this technique. Specifically, it is important to select the correct wavelengths to illuminate the cortex. Depending on the wavelength used, it will dictate the signal being measured from the brain of the animal. For example, changes can be detected by using different wavelengths for total hemoglobin and blood flow (500-599nm) and deoxyhemoglobin (600-699nm) measurement (Morone et al., 2017).
With that in mind, researchers may select to use a broadband white light source and filter individual wavelengths or use individual wavelengths from (for example) Mightex’s multi-wavelength LED sources. When using a multi-wavelength LED source, researchers are able to switch between multiple wavelengths and synchronize acquisition with the camera to detect different signals.
Lastly, many scientists will employ (Juavientt et al., 2018) a lightguide to illuminate the skull for imaging, as this provides the most control of the light direction.
Intrinsic optical imaging has its strengths and weaknesses, like many techniques. Many researchers employ this technique because it is non-invasive, requiring very little surgical intervention and minimal equipment (Juavientt et al., 2018). Intrinsic optical imaging provides a large field-of-view with relatively good spatial and temporal precision for individuals studying activity across the whole-cortex or large cortical regions (Lu et al., 2017).
However, intrinsic optical imaging lacks in certain areas. First, this technique provides measurement of global neural activity with no ability to target specific neuron populations (e.g. excitatory vs inhibitory). Second, depending on what is being studied, intrinsic imaging may have too slow temporal resolution, with seconds precision, or too low of spatial resolution, with no ability to visualize individual neurons (Juavientt et al., 2018). Lastly, intrinsic optical imaging is solely used for recording neural activity and cannot be used for manipulating neural activity to test potential causal relationships.
The limitations of intrinsic optical imaging do not necessarily require replacing the technique; instead, these limitations can be addressed by using complementary optical techniques. The integration of techniques such as fluorescent activity indicators and optogenetics can greatly benefit intrinsic optical imaging experiments.
Fluorescent activity indicators, such as genetically-encoded calcium and voltage indicators, can help overcome the spatial and temporal limitations of intrinsic imaging. These indicators are genetically expressed and identified through fluorescence emission (Grienberger & Konnerth, 2012), in comparison to intrinsic optical imaging that simply requires reflected light (Hillman et al., 2008). The genetic expression of these indicators makes them capable of identifying explicit cellular population activity.
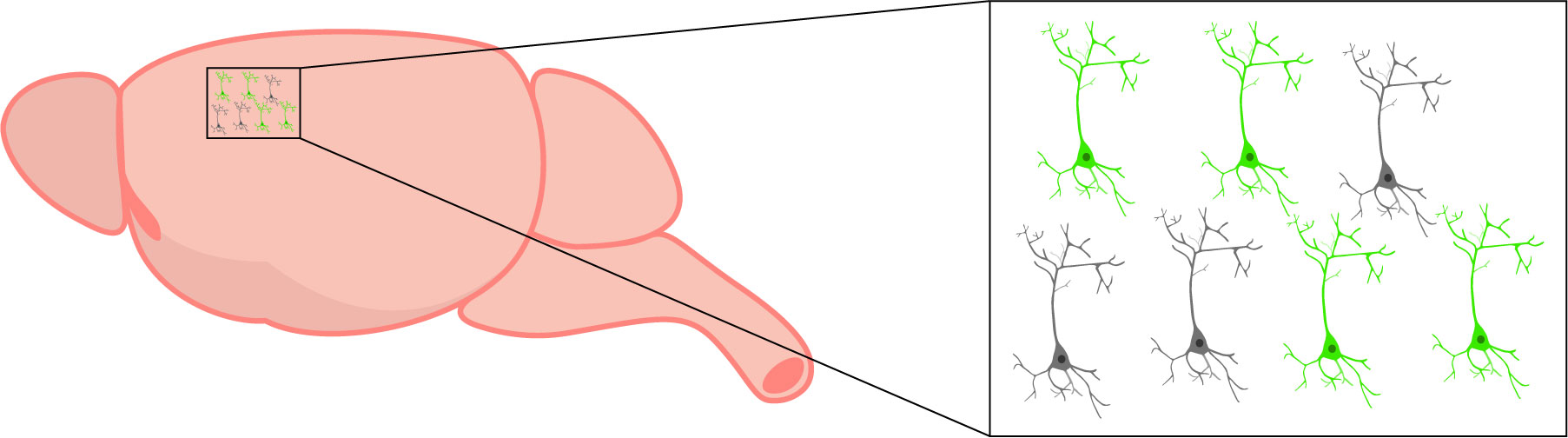
In addition, changes in fluorescence of activity indicators allows for identification of individual cells (Grienberger & Konnerth, 2012), unlike intrinsic imaging that only detects global activity. Fluorescent activity indicators also display quicker temporal dynamics (Lin & Schnitzer, 2017), which may be important depending on the experiment.
Since both techniques require light to function, they are complementary to each other and can be integrated into the same optical system. There are two potential setups for integrating fluorescent imaging indicators with optical intrinsic imaging.
First, a standard mesoscope setup can be used with both techniques. This allows for large field-of-view imaging. Intrinsic imaging may help identify regions, while fluorescent imaging indicators provide higher temporal dynamics and specific cell-type population activity changes across the cortex.
Second, one can potentially use large-scale intrinsic optical imaging to detect regional changes with a mesoscope, and combine this with high-resolution two-photon imaging (Morone et al., 2017). This combination provides the ability to not only detect large-scale changes, but also activity changes at the single-cell level to correlate global and single-cell activity.
The downside to using fluorescent activity indicators is they require optimization of expression, more intensive surgical procedures, and additional equipment. Compared to intrinsic imaging that uses the intrinsic properties of the brain, fluorescent activity indicators must be expressed in the brain of the animal (Resendez et al., 2016). This necessitates intense optimization of viral expression to achieve high imaging-quality data.
Furthermore, fluorescent indicators require surgical intervention to implant a cortical window and express the virus, making it a more invasive imaging technique (Resendez et al., 2016). Lastly, the addition of the appropriate filter set and excitation light source is necessary to both excite the indicator and image its fluorescent emission.
In summary, fluorescent activity indicators can complement intrinsic imaging experiments by providing higher temporal precision, cell-type specificity, and spatial precision while recording neural activity across the cortex.
While intrinsic optical imaging provides large-scale measurement of neural activity across the cortex, this technique is unable to manipulate neural activity, which is essential for scientists to study causal relationships between cortical connections, behaviour, and sensory responses.
The introduction of optogenetics, however, has provided scientists with an optical technique to perturb neural activity in a well-controlled manner. By using light-activated channels that can be genetically expressed in the brain, optogenetics can be used to manipulate neural activity with high temporal precision and cell-type specificity (Boyden et al., 2005).
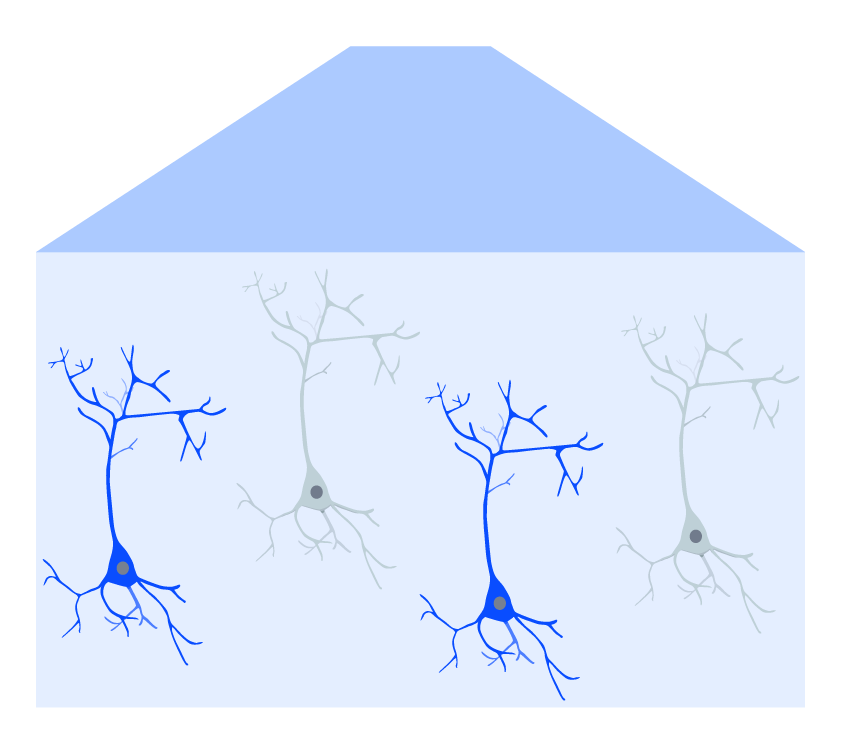
With both intrinsic optical imaging and optogenetics using light, these techniques can be integrated and complement each other to provide cortical imaging and manipulation (Nakamichi et al., 2019). Two methods of optogenetics can be performed with intrinsic imaging across the cortex using a mesoscope: widefield and targeted.
Widefield optogenetics can be used to perturb activity across the entire field of view with no spatial precision. In comparison, targeted optogenetics, based on (for example) Mightex’s Polygon DMD Illuminator, enables scientists to target individual parts of the cortex (or defined groups of neurons), in order to study how cortical areas or certain neuron populations influence cortical activity.
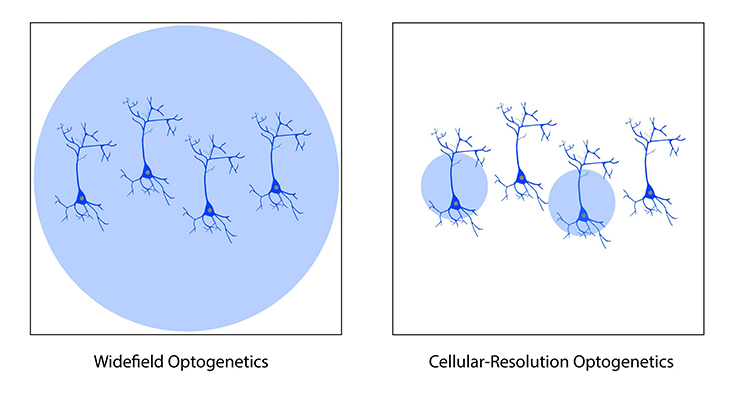
Although optogenetics is used to manipulate neural activity, it requires genetic expression and surgical invasiveness. Similar to calcium imaging, expression of the optogenetic probe may need to be optimized to achieve peak activation or inhibition of neural activity (Mei & Zhang, 2012). In addition, the implantation of a cortical window may be required to perform optogenetics, increasing the invasiveness of the technique.
The other consideration of using optogenetics with intrinsic imaging is overlapping excitation wavelengths. Depending on the experiment, both techniques may use the same excitation wavelengths; thus, intrinsic imaging may induce optogenetic activation or inhibition leading to unintended activity changes. It is, therefore, crucial to either select an optogenetic probe that has a non-overlapping excitation wavelength with intrinsic imaging or select a non-overlapping wavelength for intrinsic imaging (Emiliani et al., 2015).
Optogenetics can complement intrinsic imaging by providing the ability to manipulate neural activity with high temporal precision, cell-type specificity, and spatial precision to test causal relationships.
Intrinsic optical imaging has greatly contributed to research studying the cortex by providing a non-invasive technique to record neural activity. The development of fluorescent indicators and optogenetics can help advance and complement intrinsic optical imaging experiments by providing cell-type specificity, temporal precision, single-cell precision, and manipulation of neural activity.