Asymmetric distribution of determinants is an important mechanism for patterning cells and embryos throughout development. Whether it is the polarized localization of PAR proteins, or RNA and protein granules, the spatial anisotropy of specific molecules within cells can lead to different cell behaviours and cell fate determination. Many biochemical networks that induce and maintain polarization have been well characterized, however, Dine et al. set out to define how a physical interaction could play a significant role in the establishment and maintenance of asymmetry in living cells. The authors focus on protein phase separation, a process in which the clustering of multiple monomers can lead to the emergent cluster gaining liquid-droplet-like or aggregate-like properties, separating it from the solution, like oil droplets in water.
To investigate how phase separation could lead to asymmetry in cells, Dine et al. first turned to computational simulation analysis. Computational simulations of spatial clustering via phase separation showed that the removal of an external polarized stimulus (e.g., a spatially-defined increase in temperature) did not alter the asymmetric distribution of clusters over time, which meant that the system intrinsically maintained the polarity induced by the stimulus.
To test whether this would hold true in cells, the authors implemented an optogenetic approach to induce the dissociation of liquid-droplet-like clusters under light exposure. Dine et al. developed PixELLs, an optosystem based on PixD and PixE proteins that associate in the dark and dissociate under blue light within seconds. By fusing these proteins to intrinsically disordered regions (IDRs), the authors were able to induce phase separated protein droplets in the dark that dissociate with blue light.
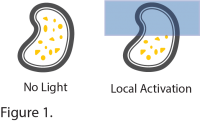 To promote asymmetric distribution of droplets in cells, the authors used Mightex’s Polygon400 pattern illuminator to restrict blue light illumination to specific regions-of-interest. Local illumination induced the dissociation of phase separated droplets within the illuminated region, while droplets fused and increased in size in the dark areas (Figure 1). Congruent with the computational simulations, the asymmetric distribution of phase separated droplets persisted after removal of the light stimulus, suggesting phase separation is a mechanism to maintain spatial memory of asymmetry over a long period of time. The authors went on to confirm that this mechanism was active in different cellular compartments including the cytosol, nucleus and membrane.
To promote asymmetric distribution of droplets in cells, the authors used Mightex’s Polygon400 pattern illuminator to restrict blue light illumination to specific regions-of-interest. Local illumination induced the dissociation of phase separated droplets within the illuminated region, while droplets fused and increased in size in the dark areas (Figure 1). Congruent with the computational simulations, the asymmetric distribution of phase separated droplets persisted after removal of the light stimulus, suggesting phase separation is a mechanism to maintain spatial memory of asymmetry over a long period of time. The authors went on to confirm that this mechanism was active in different cellular compartments including the cytosol, nucleus and membrane.
They also verified that a gradient light stimulus delivered by the Polygon400 could induce a sharply delineated boundary between regions occupied by droplets and regions where droplets dissociated. When shone with a light gradient, PixELL-expressing cells showed a clearly defined spatial distribution of droplets that persisted for a long period of time after removal of the gradient light stimulation (see here for a video). This is important as many biochemical gradients in nature are known to induce clear asymmetric distribution of determinants, suggesting phase separation could be a mechanism that translates the gradient input into persistent polarized distribution of proteins within cells.
To determine whether phase-separation-induced asymmetry of proteins could impact cellular behaviour, Dine et al. switched to a different optosystem as well as cellular context. Upon further testing, the authors decided to use the previously-developed optoDroplets system, which is a CRY2-based optogenetic tool that promotes clustering when stimulated by blue light. By fusing this system with Fibroblast Growth Factor Receptor 1 (FGFR1) – together with a myristoyl tail and IDR – the authors were able to induce clustering and phase separation of the receptor at the plasma membrane of cells. FGF signaling is an important pathway for the induction of different cellular behaviours including cytoskeletal rearrangement and cell migration, and clustering of FGFR1 is known to activate the pathway.
Since this system induces clustering under blue light, the illumination workflow with Mightex’s Polygon400 had to be modified. To induce persistent asymmetry of FGFR1 clusters, the Polygon400 was first used to locally stimulate a subcellular region-of-interest and then illumination was switched to global activation. Under this illumination protocol, cells maintained spatial memory of cluster distribution. Local activation of the FGFR1 led to cell constriction in the illuminated areas, which persisted when illumination was switched to global activation, suggesting asymmetric distribution of receptors via phase separation can indeed lead to persistent physiological changes in a cellular context.
With this elegant approach, Dine et al. showcases once again the power of patterned illumination with Mightex’s Polygon400 and optogenetic-based tools in the dissection of cellular mechanisms.
To learn more about the Polygon400, click here. Or, read our white paper comparing tools for cell biology optogenetics.
To read the full paper published in Cell Systems, click here.
Dine, E., Gil, A.A., Uribe, G., Brangwynee, C.P., & Toettcher, J.E. (2018). Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Systems.



