Optogenetic induction of long-term potentiation at excitatory synapses onto hippocampal somatostatin interneurons and its regulatory effect on CA1 local circuit
Azam Asgarihafshejani, Isabel Laplante, & Jean-Claude Lacaille
Department of Neurosciences, Université de Montréal
Hippocampal CA1 somatostatin interneurons (SOM-INs) are dendrite-projecting interneurons. Excitatory inputs from pyramidal cells onto SOM-INs show an mGluR1a-mediated form of long-term potentiation (LTP) which regulates metaplasticity of the CA1 circuit. A critical SOM-IN function is the differential regulation of afferents onto pyramidal cells: via distal dendritic inhibition, they downregulate activity and LTP in the temporo-ammonic pathway (TA); whereas via proximal dendritic disinhibition, they upregulate activity and LTP in the CA3-CA1 Schaffer collateral pathway (SC).
The aim of this study is to determine the function of long-term synaptic plasticity induced optogenetically in SOM-INs, establish the conditions to induce synaptic plasticity in SOM-INs by optogenetics in slices, identify the molecular and cellular mechanisms involved, determine if optogenetically induced LTP in SOM-INs regulates CA1 network plasticity in slices,and ultimately determine if optogenetic induction of SOM-IN LTP in vivo regulates hippocampal-dependent learning and memory.
For optogenetic activation of pyramidal cell excitatory inputs to SOM-INs, we injected AAV-CaMK2a-ChR2 (E123T/T159C)-mCherry in CA1 of SOM-Cre-eYFP mice. Whole-cell recordings showed that single optogenetic stimulation in oriens-alveus (Mightex, Polygon400DSI-E-0470-0560-0656-000.5mW, 470nm) elicited EPSPs (EPSPopto) in SOM-INs in slices. Optogenetic theta burst stimulation (TBSopto; bursts of 4 pulses at 80Hz, repeated 5 times at 300 ms-1, given 3 times at 30 sec-1) produced LTP of EPSPopto. LTP was absent with low frequency TBSopto or in untetanized cells. LTP of EPSPopto was mediated by mGluR1a (blocked by LY367385) and mTORC1 (absent in conditional SOM;Rptor-/-mice). Optogenetically-induced SOM-IN LTP is thus analogous to electrically-evoked LTP. We found that TBSopto produced LTP of electrically-evoked EPSPs (EPSPelect) which was also mediated by mGluR1a and mTORC1. Finally TBSopto by whole field fiber illumination (300 μm diameter; Thorlabs) elicited similar LTP of EPSPopto.
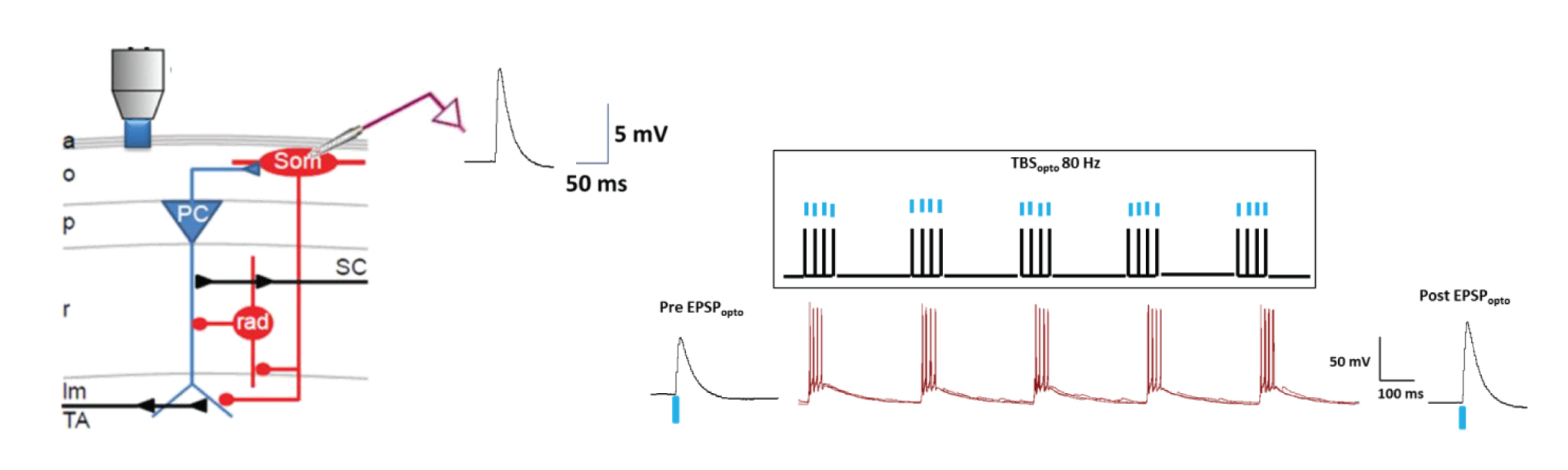
Figure 1. Optogenetic theta burst stimulation (TBSopto; bursts of 4 pulses at 80Hz, repeated 5 times at 300 ms-1, given 3 times at 30 sec-1) produced LTP of EPSPopto.
Next we tested if wholefield TBSopto,that produce mGluR1a-mediated LTP at SOM-IN synapses,would result in a differential regulation ofSC-and TA-LTP using field potential recordings in slices.At 25 min after TBSopto, LTP of fEPSPs inducted by weak theta burst stimulation (wTBS; 2bursts of 4 pulses at 100 Hzwith200 ms interburst interval) was enhanced in the Schaffer collateral pathway and depressed in the TA pathway, relative to wTBS alone.
Thus, mGluR1a-and mTORC1-mediated LTP of CA1 pyramidal cell excitatory synapses onto SOM-INs is induced by optogenetic stimulation and is sufficient to differentially regulate metaplasticity of Schaffer collateral and temporo-ammonic pathways of CA1 area.The protocol developed in this study for optogenetic induction of LTP in SOM-INs provides a tool to determine in vivo the role of SOM-IN synaptic plasticity in hippocampal-dependent learning and memory.
Author: Azam Asgarihafshejani, Université de Montreal
Bio: Azam Asgarihafshejani (Asgari) received her PhD in Physiology from Tarbiat Modares University in Tehran, Iran where her research focused on using electrophysiological approaches to study epilepsy. She then completed a postdoctoral fellowship in the Delaney lab in the Biology department at the University of Victoria studying a mouse model of Rett Syndrome. Before joining the Woodin lab Azam also completed a second post-doctoral fellowship in the Lacaille lab at Université de Montreal where she complemented her previous experience with novel approaches using cell-specific conditional knockout mouse lines, optogenetics, and patch clamp electrophysiology



