Photopharmacology — New tools for optical control in biology and medicine
Andrew Woolley
Department of Chemistry, University of Toronto
The new field of optogenetics has excited a large number of scientists because it enables new ways of studying the brain and other complex biological systems. In optogenetics, naturally-occurring, light sensitive ion channels are expressed using genetic methods in specific neurons. These channels confer light sensitivity of the targeted neurons. Light pulses delivered to these neurons then control their firing. Through the work of a number of researchers optogenetic methods have recently been extended to the control of proteins other than ion channels. For example, designed light controlled proteins can be used to confer light sensitivity on transcription factors, thereby producing optical control of gene expression. However, each of these optogenetic methods requires the introduction of new genetic material into the host organism. While extraordinary powerful as a research tool in animals, this need for introduction of new genetic material complicates the application of optogenetic approaches as a means to treat diseases in humans.
Photopharmacology, in which a small molecule drug is made light sensitive is an alternative means to confer light sensitivity on biological targets. In general, drugs used in humans are either small molecules or proteins; these are administered transiently and avoid most of the complications associated with gene therapy. The basic concept of photopharmacology has been around for some time but the idea has recently seen a major resurgence due to clever molecular design and improvements in optical delivery methods. Photo-pharmaceuticals have already become a reality as a powerful research tools, and have real potential for clinical applications. A recent overview for the general public appeared in the The Economist: http://www.economist.com/news/science-and-technology/21657352-optical-switching-may-abolish-side-effects-cancer-drugs-colourful.
There are a variety of approaches that can be taken to producing a photo-pharmaceutical. In general researchers have started with a known drug, or the known small molecule ligand for a given biological target. Then have then chemically modified the molecule to incorporate a light sensitive component. Examples include combrestatin, an anti-cancer drug isolated from the bark of the South African Bush willow and glutamate, a naturally occurring neurotransmitter in the human brain.
Combrestatin binds to microtubules, polymeric proteins that are essential to cytoskeleton production. If cancer cells cannot make cytoskeleton, they cannot effectively grow and divide. Thorn-Seshold et al made an analogue of combrestatin that incorporates the azobenzene molecule (Fig. 1), a well-known light sensitive chemical group.3 Shining violet (390-430 nm) light at this compound, designated ’photostatin’, changes its conformation to the cis isomeric form, which is 250 times more cytotoxic than the trans isomeric form. Shining green light (500-530 nm) returned the molecule to its less active trans isomeric form. Optical control of cytotoxicity offer the potential for targeted chemotherapy.
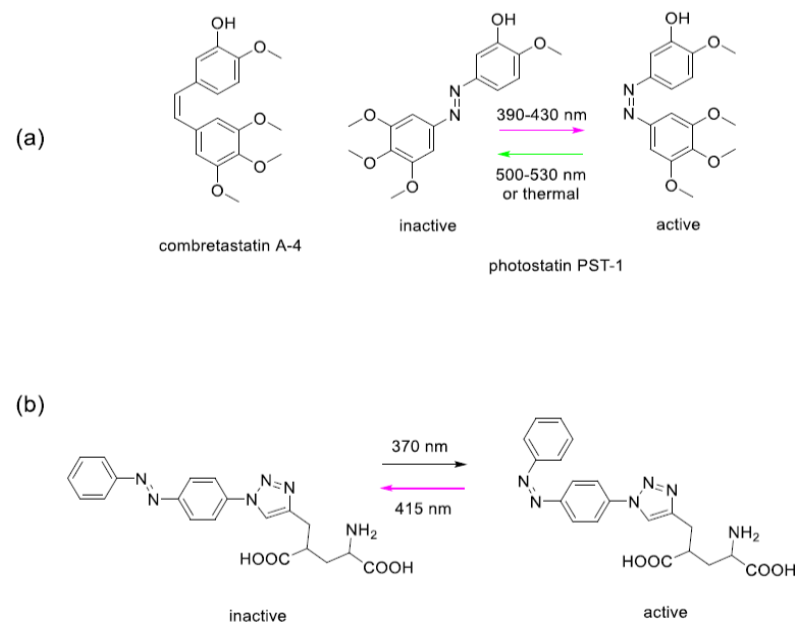
Figure 1. Photo-control of an anticancer drug (a) and a neurotransmitter (b). Glutamate is a naturally occurring neurotransmitter in the human brain. When glutamate is released by certain neurons, it binds to receptors on adjacent neurons and causes those neurons to fire. Too much firing or too little firing can be associated with a wide range of neurological disorders including ischaemia-related cell death, Alzheimer’s, Huntington’s and Parkinson’s diseases, as well as schizophrenia and autism spectrum disorders. Trauner and colleagues recently reported a light switchable glutamate analogue, azobenzene-triazole-glutamate (ATG), which is specific for a certain subtype of glutamate receptors called N-methyl-D-aspartate receptors (NMDAR).4 Again, the molecule was made photosensitive by introducing an azobenzene group. ATG is inactive in its dark-adapted trans-isoform, but can be converted into its active cis-isoform with UV light (Fig. 1). These studies demonstrate the power of photopharmacology. However, in our view, a key feature that will determine the ultimate usefulness of photopharmacology for application in vivo, and particularly in humans, is the wavelength of light needed to cause the isomerization events. Scattering and absorption of light by tissue is severe in the UV and visible ranges. Only far red and near infrared light can penetrate significant distances (Fig. 2) without the use of fibre optics. We have thus focussed on the development if azobenzene based photoswitchable compounds that can operate using red, far-red and near infrared light.
By activating the compounds with light only at the location of a tumour it may be possible to significantly reduce serious off-target effects associated with systematic administration of non-switchable versions of the drugs. We have found that introducing substituents at all four ortho positions leads to azo compounds with a number of unusual properties that are useful for in vivophotoswitching. When para positions are also substituted with amino groups, ortho methoxy groups greatly stabilize the azonium form of thecompounds, in which the azo group is protonated. Azonium ions absorb strongly in the red region of thespectrum and can reach into the near-IR.These azonium ions can exhibit robust cis—trans isomerization in aqueous solutions at neutral pH.5.
We have been systematically designing, synthesizing and testing the properties of these switches using a range of high power LED sources from Mightex Systems(BLS-LCS-0740-10-22 and BLS-SA04-US). Our latest results have been reported in Chemical Communications 2015, 51, 12981-4.6 Figure 3 shows an azobenzene based switch developed during these studies that isomerizes with >700 nm light.
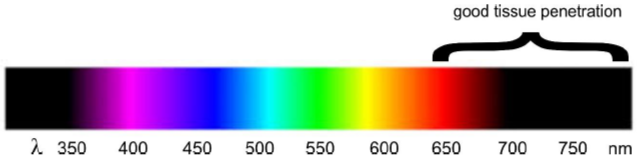
Figure 2. The penetration depth of light through tissue is much greater in the far red and near IR regions.
We have found that introducing substituents at all four ortho positions leads to azo compounds with a number of unusual properties that are useful for in vivo photoswitching. When para positions are also substituted with amino groups, ortho methoxy groups greatly stabilize the azonium form of thecompounds, in which the azo group is protonated. Azonium ions absorb strongly in the red region of thespectrum and can reach into the near-IR. These azonium ions can exhibit robust cis—trans isomerization in aqueous solutions at neutral pH.
Some of these newly developed photoswitches can be used in whole blood and show promise for effective use in vivo. It is hoped they can be combined with appropriate bioactive targets to realize the potential of photopharmacology. Together with high power long wavelength light sources and spatial patterning, these photo-pharmaceuticals may one day be used clinically and allow a new degree of control over drug action in human patients.
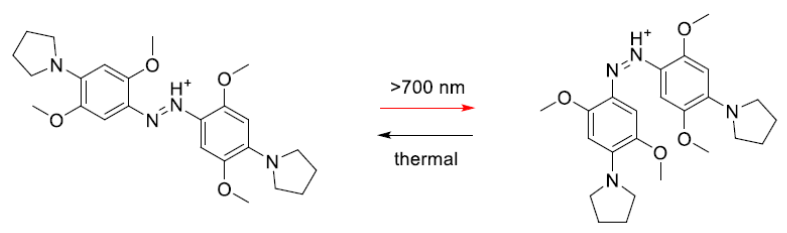
Figure 3. A newly discovered azobenzene base photoswitch that isomerizes with near IR light.
References:
(1) Moglich, A.; Moffat, K. Engineered photoreceptors as novel optogenetic tools. Photochem. Photobiol. Sci. 2011, 9, 1286-1300.
(2) Ali, A. M.; Reis, J. M.; Xia, Y.; Rashid, A. J.; Mercaldo, V.; Walters, B. J.; Brechun, K. E.; Borisenko, V.; Josselyn, S. A.; Karanicolas, J.; Woolley, G. A. Optogenetic Inhibitor of the Transcription Factor CREB. Chem. Biol. 2015, 22, 1531-1539.
(3) Borowiak, M.; Nahaboo, W.; Reynders, M.; Nekolla, K.; Jalinot, P.; Hasserodt, J.; Rehberg, M.; Delattre, M.; Zahler, S.; Vollmar, A.; Trauner, D.; Thorn-Seshold, O. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 2015, 162, 403-411.
(4) Laprell, L.; Repak, E.; Franckevicius, V.; Hartrampf, F.; Terhag, J.; Hollmann, M.; Sumser, M.; Rebola, N.; DiGregorio, D. A.; Trauner, D. Optical control of NMDA receptors with a diffusible photoswitch. Nat. Commun. 2015, 6, 8076.
(5) Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A. A.; Woolley, G. A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662-2670.
(6) Dong, M.; Babalhavaeji, A.; Hansen, M. J.; Kalman, L.; Woolley, G. A. Red, far-red, and near infrared photoswitches based on azonium ions. Chem. Commun.2015, 12981 – 12984.


