Reclusive Chandeliers: Dentate Axo-axonic Cells are Functionally Isolated Early After Status Epilepticus
Archana Proddutur
University of California Riverside
The parvalbumin (PV)-containing interneurons in the hippocampal dentate gyrus include fast-spiking basket cells that project to the soma and axo-axonic cells that innervate the axon initial segment of granule cells forming distinctive axonal cartridges. Due to their potential role in regulating network excitability and oscillations, PV interneurons are often proposed as targets for therapeutic intervention to curb ongoing seizure activity. Using in-vivo optogenetic activation of PV interneurons few studies have reported successful seizure termination. However, a large body of in-vitro studies suggests that activation of PV interneurons exacerbates seizures. This ambiguity in outcomes could, in part, arise from differential pathophysiological changes in inhibition mediated by axo-axonic cells from that of basket cells. Therefore, identifying unique changes in basket versus axo-axonic cell mediated inhibition is vital before attempting to develop strategies to target PV neurons for seizure treatment.
The parvalbumin (PV)-containing interneurons in the hippocampal dentate gyrus include fast-spiking basket cells that project to the soma and axo-axonic cells that innervate the axon initial segment of granule cells forming distinctive axonal cartridges. Due to their potential role in regulating network excitability and oscillations, PV interneurons are often proposed as targets for therapeutic intervention to curb ongoing seizure activity. Using in-vivo optogenetic activation of PV interneurons few studies have reported successful seizure termination. However, a large body of in-vitro studies suggests that activation of PV interneurons exacerbates seizures. This ambiguity in outcomes could, in part, arise from differential pathophysiological changes in inhibition mediated by axo-axonic cells from that of basket cells. Therefore, identifying unique changes in basket versus axo-axonic cell mediated inhibition is vital before attempting to develop strategies to target PV neurons for seizure treatment.
Our aim is to understand how specific synapses from axo-axonic cells to granule cells are altered following pilocarpine-induced temporal lobe epilepsy in mice. Age-matched PV cre-Channelrhodopsin transgenic control mice and one-week post pilocarpine treatment mice were used. Mightex Polygon DMD was used to precisely activate single neurons and evaluated synaptically coupled axo-axonic cell→granule cell pairs to determine whether the unitary IPSC (uIPSC) are altered after pilocarpine treatment. As described above, prior to recordings, we identify single axo-axonic neurons in the inner molecular layer of dentate gyrus under YFP epifluorescence and mark a circular ROI ~50um for DMD illumination via TTL based trigger from pClamp software. Then we performed whole-cell patch clamp recordings in granule cell under voltage clamp mode and precisely activated single neuron with 5ms pulse width, 473nm blue LED for 10 trails. Single cell activation precisely via DMD Polygon, is especially useful where dual patch or multi-patch clamp is used to study cell specific changes at synapse level.
Note: control experiments in which ROIs around non-ChR2/EYFP cells were activated did not result in postsynaptic response in granule cells.
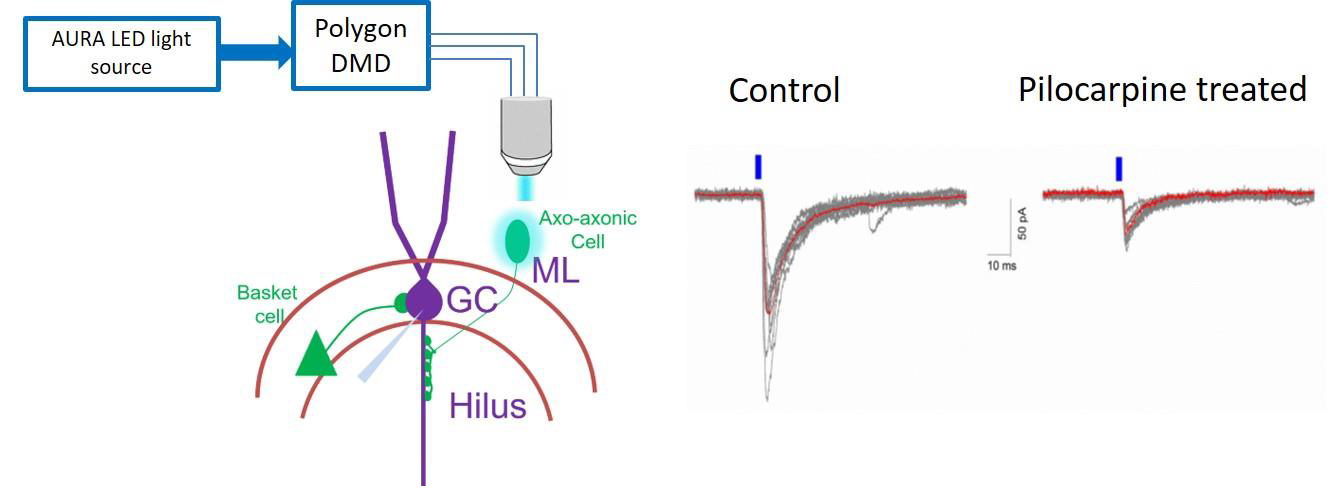
Figure 1. Schematic on left shows optogenetic activation of single axo-axonic cell via DMD and whole-cellvoltage-clamp recordings in granule cell (GC). Representative traces on right shows uIPSCs in granule cells evoked by brief stimulation of single presynaptic axo-axonic cell in control and one-week post pilocarpine treatment (average trace is overlaid in red).
Author: Archana Proddutur
Bio: I received Ph.D. from Rutgers University under the guidance of Dr. Viji Santhakumar. My primary research goal is to understand how cell-specific inhibition modulates the excitability of healthy and epileptic networks. In the hippocampal dentate gyrus, using cell-specific optogenetics along with patch-clamp electrophysiology, I examined physiological and functional differences in two parvalbumin subtypes, namely basket cells and axo-axonic cells in control and seizure conditions. Also, I used biologically realistic computational network models to enhance our understanding of transient changes in synaptic activity.



