Published on 2025/02/24 Research powered by Mightex’s Polygon1000 

Chen, S. C., Zeng, N. J., Liu, G. Y., Wang, H. C., Lin, T. Y., Tai, Y. L., … & Lin, Y. C., Precise Control of Intracellular Trafficking and Receptor‐Mediated Endocytosis in Living Cells and Behaving Animals. Advanced Science, 11(45), 2405568 (2024).


All eukaryotic cells require intracellular trafficking of molecules for all critical cellular activities. This requires use of complex transport networks that are regulated in both temporal and spatial domains by various protein families1. Historically, numerous chemical reagents producing inhibition or modification of intracellular pathways have been used to understand the role of these regulator proteins in these networks2. However, almost all of these methods focus on temporal rather than spatial perturbation to elucidate proper functioning and putative roles of regulator proteins. As such, this paper puts forward a novel and highly versatile spatiotemporal method, called RIVET (Rapid Immobilization of target Vesicles on Engaged Tracks) for manipulating target transport pathways that allow scientists to study how intracellular trafficking networks coordinate their functions in order to maintain cellular homeostasis (figure 1).
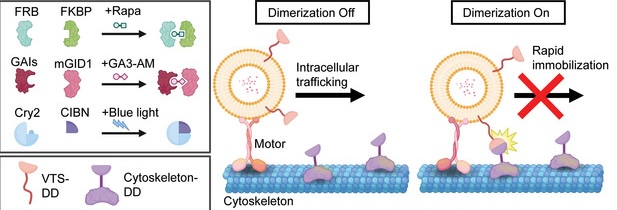
Figure 1: Schematic of RIVET demonstrating opto-inducible dimerization. Adapted from Chen et al., 2024.
Chen and colleagues first developed an optically-inducible tool based on the well established mechanism of intracellular trafficking along cytoskeletal structures by microtubule action. The method they chose uses rapamycin induced dimerization between FKBP (FK506-binding protein) and FRB (FKBP-rapamycin binding domain) along with a yellow fluorescent protein tag (mNeon). Together, this construct allows fusion with target proteins in a vesicle of interest without creating any changes to regular vesicular dynamics, as tested by several verification experiments highlighting the highly specific and reversible nature of the construct (figure 2).
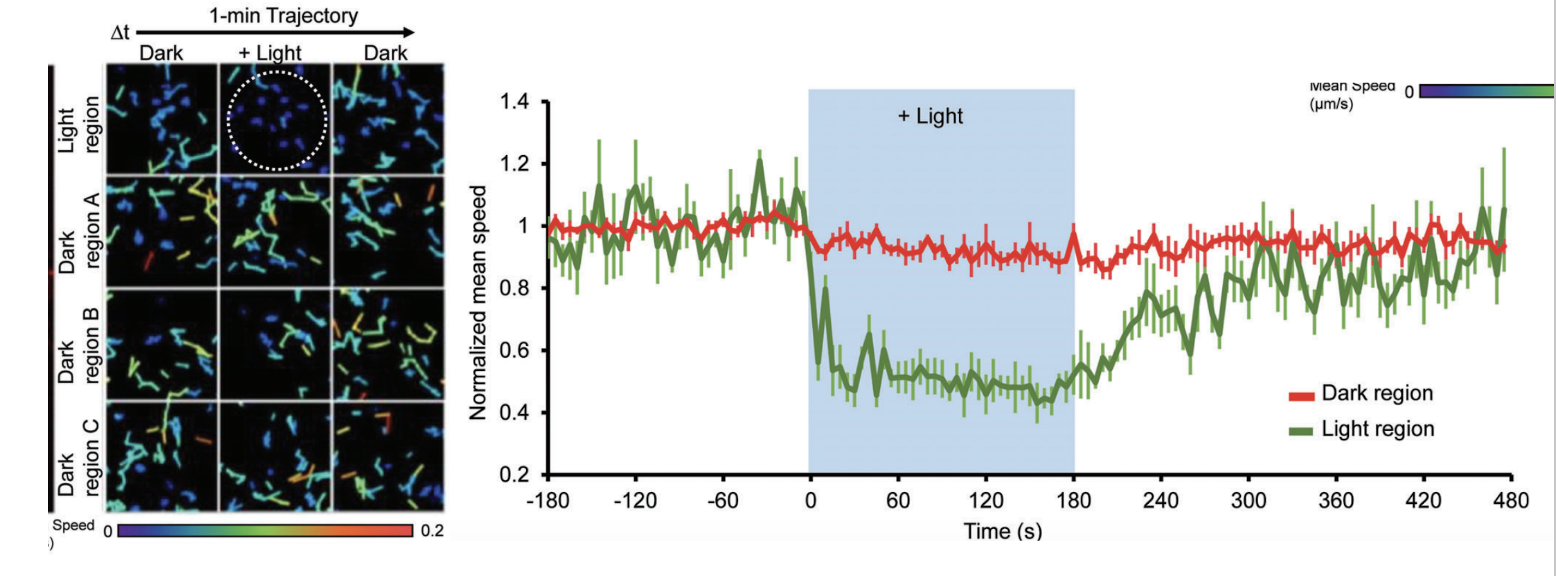
Figure 2: Demonstration of opto-induced RIVET action in microtubule structure and intracellular movement. Adapted from Chen et al., 2024.
RIVET was shown to inhibit the delivery of specific molecules between various intracellular locations. Rapamycin treatment, induced with 488nm light attenuated this process. Further, vesicles of interest (VOIs) were perturbed from their expected trajectory within cells by utilising optogenetics, again using blue (488nm) light this time in a spatially specific manner. Lastly, the authors showed immobilization of specific microtubules involved in vesicular movement impacting intracellular signaling drastically. Taken together, these demonstrations of the powerful effects of RIVET highlight the potential usefulness of this optically-inducible tool for exploring intracellular networks.
The Mightex Polygon 400 Pattern Illuminator was employed in this paper as a vital tool for spatially targeted optoactivation of the RIVET construct within both cell culture and C.elegans in vivo live cell imaging preparations.
References
1. Schwartz T. Functional insights from studies on the structure of the nuclear pore and coat protein complexes. Cold Spring Harbor Perspectives in Biology. 2013 Jul;5(7):a013375
2. Gleeson, P.A., Lock, J.G., Luke, M.R. and Stow, J.L. (2004), Domains of the TGN: Coats, Tethers and G Proteins. Traffic, 5: 315-326.
To read the full publication, please click here.



