Published on 2024/09/23 Research powered by Mightex’s Polygon1000 
 Brumbaugh-Reed, E. H., Gao, Y., Aoki, K., & Toettcher, J. E., Rapid and reversible dissolution of biomolecular condensates using light-controlled recruitment of a solubility tag, Nature communications, 15(1), 6717 (2024).
Brumbaugh-Reed, E. H., Gao, Y., Aoki, K., & Toettcher, J. E., Rapid and reversible dissolution of biomolecular condensates using light-controlled recruitment of a solubility tag, Nature communications, 15(1), 6717 (2024).


Introduction
Biomolecular condensates are clusters of membrane-less organelles within eukaryotic cells that carry out specialised functions (1,2). They are created by groups of proteins and nucleic acids forming weak multivalent bonds and assembling into dense phases surrounded by dilute fluid (Figure 1.). Biomolecular condensates play fundamental roles in a plethora of biological processes, including stress responses, immune regulation and transcription (3). Further, aberrant processes in biomolecular condensates are implicated in numerous disease states, including neurodegenerative conditions and cancer (4).
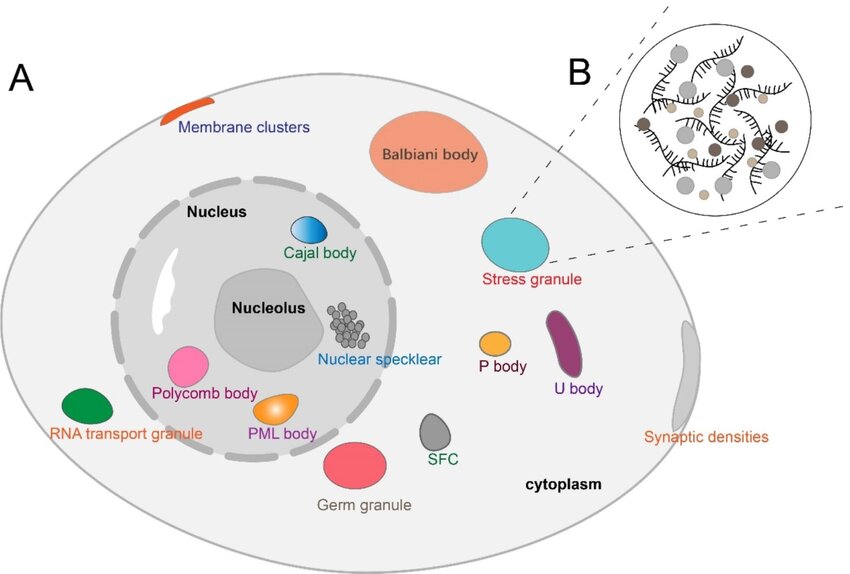
Figure 1. Schematic of biomolecular condensate formation in eukaryotic cells. Reproduced from Nat. Rev. Mol. Cell Biol. 18, 285–298; 2017, Springer Nature Limited.
The study of biomolecular condensates and their roles in healthy and disease state cell functions has proved challenging (5). While biochemical tools exist for inducing phase separation and formation of biomolecular condensates, few tools are available that interrogate the specific role of individual biomolecular condensates by targeted dissolution. The tools that do exist are pharmacological and are accompanied by numerous disadvantages. Therefore, there remains a need for novel molecular condensate dissolving tools that are precise, rapid, and reversible. One putative target for such a tool is the maltose binding protein (MBP) which is a widely used purification tag that is capable of increasing solubility of various proteins which undergo phase separation. Brumbaugh-Reed and colleagues sought to develop a new tool (opto-MBP) based on light-inducible recruitment of maltose-binding protein (MBP) to increase solubility of condensates temporally.
In this paper, the authors lay out the development and proof of principle basis of their novel tool, Opto-MBP. Opto-MBP provides optogenetically-controlled induction of biomolecular condensate reversible dissolution, with high temporal and spatial specificity. Opto-MBP provides an exciting step forward in exploring the diverse roles of biomolecular condensates in healthy and disease states.
Identifying a model biomolecular condensate and putative optogenetically solubilizer tool
First the authors isolate which specific biomolecular condensate to use for demonstrating the function of the dissolution tool. They focused their efforts on the intrinsically disordered region of Fused in Scarcoma (FuS), which is a condensate that has been well studied in chemogenetic and optogenetic literature. In order to verify that this biomolecule creates condensate, the authors generated a construct composed of three FUS repeats, a light-inducible dimer domain, and a chemogenic recruitment domain. When introduced to HEK293T cells, this construct successfully formed condensate droplets in the cellular nuclei, thus verifying the FuS as an ideal model condensate for the development of the opto-tool.
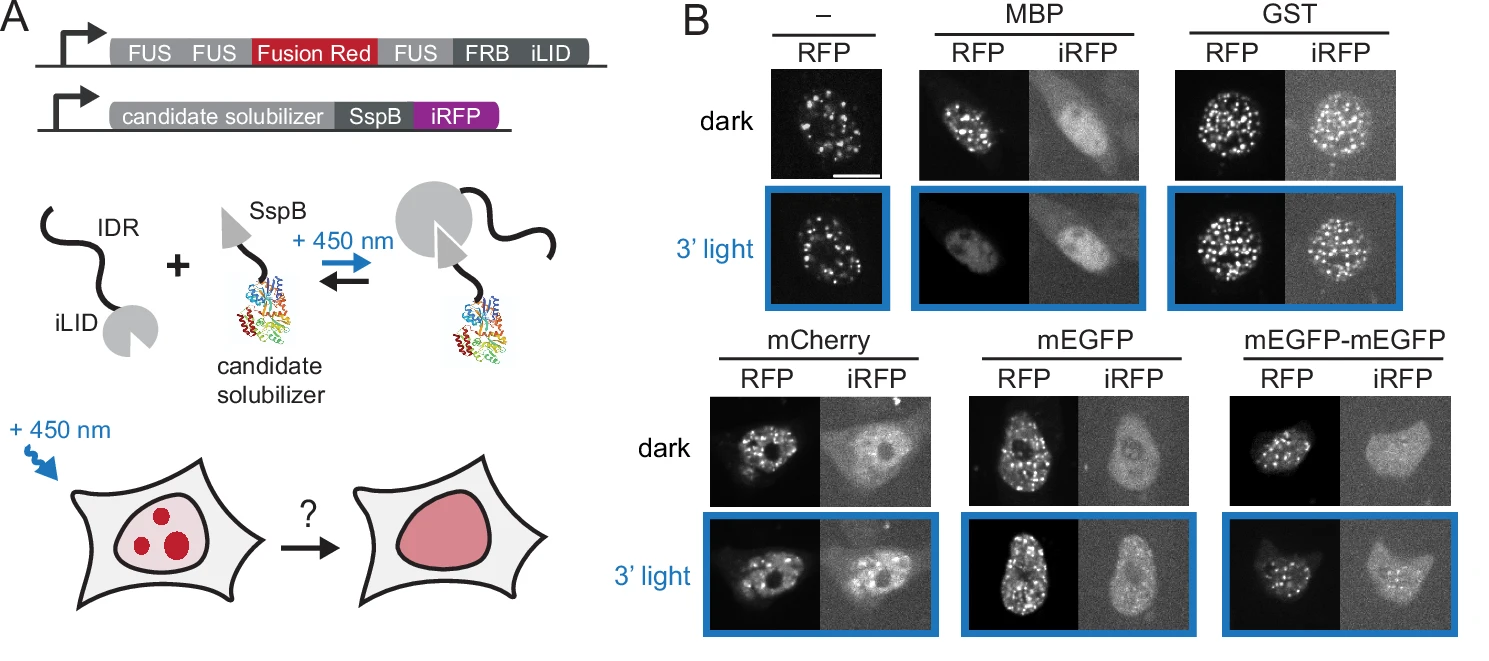
Figure 2. Schematic of opto-tool construct and application of 450nm LED light. Adapted for Brumbaugh et al.
Next, Brumbaugh-Reed sought to determine whether optogenetic recruitment of the light-inducible solubilizer domain of this 3-FuS construct could induce dissolution of the FuS components. Using MBP combined with 450nm LED illumination as the putative driver of optogenetics-induced dissolution, the light-inducible dissolution effects were isolated to only condensates of the FuS construct that contained the solubilizer domain (Figure 2.). Further, quantification of the FuS construct showed that following exposure to 450nm light, there was comparable dissolution to that induced by a well established pharmacological dissolving agent (hexandiol), thus suggesting that the optogenetic-domain was capable of producing robust and reliable dissolution of the FuSD condensate.
Using the Mightex Polygon to demonstrate the spatiotemporal precision, rapid, and reversible dissolution of condensates by opto-MBP
For an optogenetic tool for condensate dissolution to be widely adopted and beneficial to the research community, it must be able to induce rapid and reversible changes in condensate concentrations and reverse phase separation. This control on condensate dissolution must also have high temporal and spatial precision.
To demonstrate these facets of opto-MBP, Brumbaugh-Reed and colleagues projected patterned light onto their HEK293T cells using the Mightex Polygon coupled to the Nikon Eclipse Ti2 spinning disk confocal microscope. The authors used the Polygon in a dithering mode in order to achieve temporal grayscale patterning of their region of interest. Such that over time, the ROI projected onto their sample cells was illuminated with 50% intensity and 50% opacity (~200mW/cm2) over 1 second acquisitions spaced 10 seconds apart. Using this illumination strategy, the optogenetic dissolution capabilities of opto-MBP were successfully characterized, with 5 second exposure to 450nm patterned LED illumination resulting in complete condensate dispersion over a 13 second timeframe (see figure 3) . These results demonstrate that MBP-induced dissolution of biomolecular condensates can be achieved rapidly and precisely.
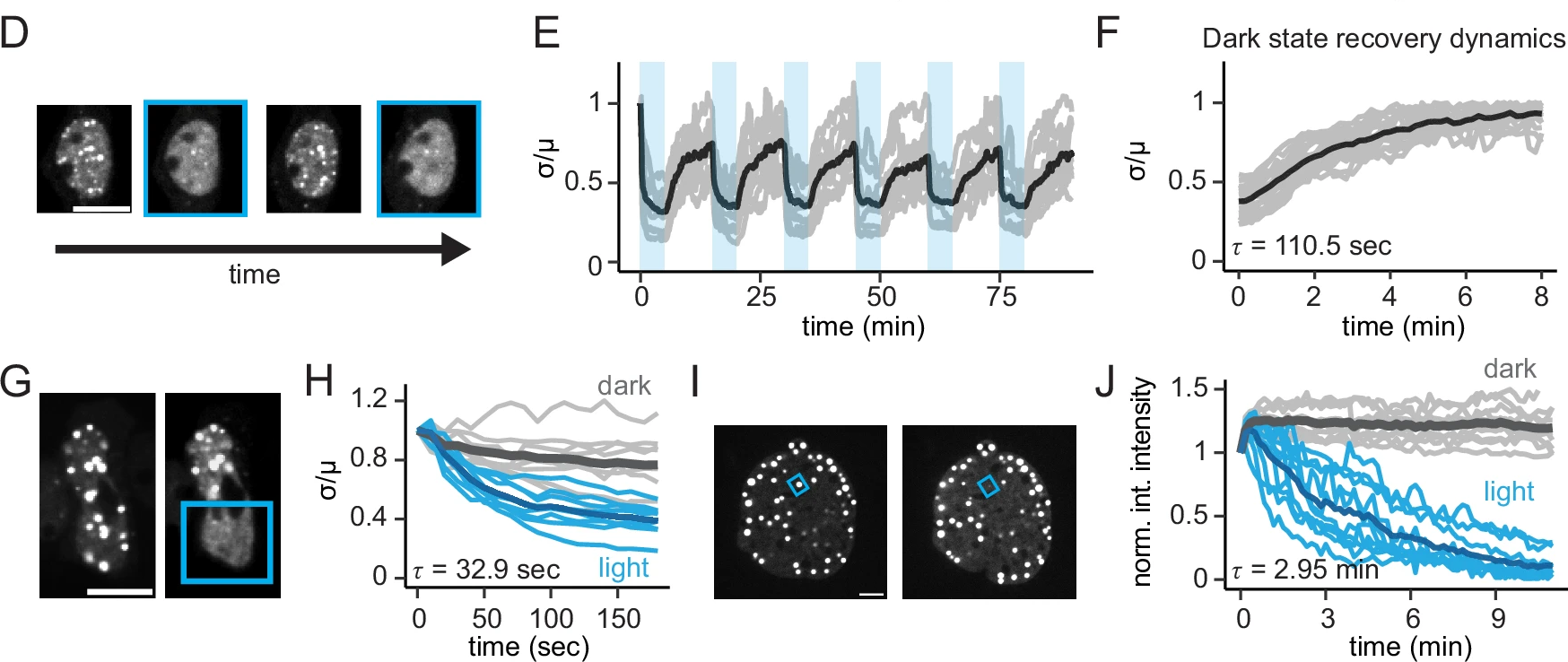
Figure 3. Targeted photostimulation and spatially isolated phase separation via opto-MBP.
To isolate the reversible dissolution of the 3-FuS condensate, HEK293T cells expressing 3-FuS were repeatedly moved between light and dark conditions while condensation concentrations were measured over time. Droplet formation was slow in dark conditions, with exposure to light inducing rapid condensate dissolution, with a complete cycle of formation and dissolution occurring on a timeframe of approximately 110 seconds. This finding was consistent with prior literature demonstrating the expected timeframe for droplet formation. Further, the repeated formation of droplets in dark conditions confirmed the reversible nature of the opto-MBP light-induced condensate dissolution.
Taken together, these findings demonstrate the opto-MBP to be a robust, accurate, and reversible tool for optogenetic induction of biomolecular condensate dissolution.
Testing the spatial limits of the opto-MBP
In order to characterize the limits of the opto-MBP capabilities, the authors next tested whether a single droplet of FuS could be dissolved using targeted photostimulation. In NIH3T3 cells that expressed the optogenetically-targetable FUs-CHOP oncoprotein. This particular system was chosen due to its larger size and stability compared to the 3-FuS and HEK293T cells, with successful dissolution reported, demonstrating powerful sub-cellular control of this opto-tool. Interestingly, the timeframe for such targeted dissolution was slower at 30 seconds to 3 minutes, compared to the previous findings of 13secons for whole cell illumination (see figure).
Generalization of opto-MBP to other condensates
In order for this optogenetically-inducible condensate dissolution tool to be a worthwhile contribution to the field, its capabilities outlined above must generalize to other condensates beyond FuS. Indeed, the authors demonstrated that they were able to generate similar results with EWS and TAF15 constructs as well as other non-FET family members, underscoring the versatility and broad range application of the opto-MBP introduced in this paper.
Implication for disease states
A growing body of literature demonstrates the need for mechanisms to study phase separation in disease states, as condensates may be functionally implicated in the development of diseases including cancer. To that end, Brumbaugh and colleagues sought to apply their optoMBP tool to the oncogene fusion protein FuS-CHOP. FUS is active in RNA metabolism and CHOP is a stress induced transcription factor. FUS-CHOP is produced by a chromosomal translocation of the N terminus section of FUS, resulting in DNA damage and formation of Myxoid liposarcoma.
To apply the optoMBP tool to FUS-CHOP dissolution, FUS-CHOP/opto-MBP cells were treated with doxycycline to induced FUS-CHOP expression. The cells were subsequently exposed to 450nm light followed by bulk RNAseq to assess transcriptional changes in the replicates. Differential expression analysis elucidated expression changes across over 800 genes, demonstrating the effectiveness of the DOX method in triggering transcriptional modifications.
Upon exposure to blue light, the opto-MBP cells showed widespread reverted gene expression of FUS-CHOP and cellular states perturbed back to basal state, indicating the opto-induced dissolution of the FUS condensate produced meaningful changes in the CHOP action.
Conclusion
Together these findings outline a novel and much needed opto-tool for the dissolution of biomolecular condensates that is precise, rapid, and reversible. Further, this paper outlined proof of principle across multiple condensate types as well as the application to a disease-state relevant onco-protein.
References
1. Dignon, G. L., Best, R. B., & Mittal, J. (2020). Biomolecular phase separation: from molecular driving forces to macroscopic properties. Annual review of physical chemistry, 71(1), 53-75.
2. Yoshizawa, T., Nozawa, R. S., Jia, T. Z., Saio, T., & Mori, E. (2020). Biological phase separation: cell biology meets biophysics. Biophysical reviews, 12(2), 519-539.
3. Shin, Y., & Brangwynne, C. P. (2017). Liquid phase condensation in cell physiology and disease. Science, 357(6357), eaaf4382.
4. Su, X., Ditlev, J. A., Hui, E., Xing, W., Banjade, S., Okrut, J., … & Vale, R. D. (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science, 352(6285), 595-599.
5. Alberti, S., Gladfelter, A., & Mittag, T. (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell, 176(3), 419-434.
To read the full publication, please click here.



