Published on 2024/01/08 Research powered by Mightex’s Polygon1000 

Bingzhi Wu and Sambeeta Das, Upstream mobility and swarming of light activated micromotors. Materials Advances, (2024).


Introduction
Micromotors are small particles that can autonomously propel themselves in a particular direction. Biological micromotors pose potential use for “Lab-on-a-chip”, cell sorting, and drug delivery applications across many biomedical fields. Most research to date has focused on the development and proof of principle of individual micromotors (references). However, individual micromotors can only carry a limited payload, and are restricted in the force they can exert. As such, the application of such technology is thwarted.
Recent advances have focused on using a micromotor “swarming” approach where many micromotors can be controlled together, overcoming many of the limitations of single micromotor approaches. In particular, swarming micromotors are able to exert higher force, carry greater payload, and travel against the flow of the liquid in which they are held. These features are important for transport of molecules in blood flow and through various media in microfluidic devices. As such, their promise for biomedical applications is broader and more tangible.
Micromotor swarms are generated by the application of various forces, including magnetic fields, chemical effects, electrical fields, thermal gradients, and acoustic waves (ref). However, all of these approaches require relatively high concentrations of chemicals, such as hydrogen peroxide, to propel their movement. These chemical concentrations unfortunately cause toxicity in living tissues, thus limiting the application of such methods for biological applications (Dong et al., 2016; 33). As such, a new approach with greater bio-compatibility is required to shift forward this technology for use in the biomedical field. With this goal in mind, Wu and colleagues have developed a light-driven technique to instigate micromotor swarming.
Wu and colleagues first used an electron-beam evaporation technique to coat titanium dioxide (Ti02) micromotors in a nickel-iron alloy, allowing the micromotors to become magnetically responsive. This process was then followed by adding layers of platinum and silver in order to increase the light-inducible movement properties of the micromotors. The micromotors were then placed into 35um x 16um microfluidic devices, generated via a standard photolithography process (ref). The Mightex Polygon was then used with a 365nm UV LED (500mW/cm2) to provide patterned illumination, allowing the micromotors to become mobile. A magnetic field was then applied to create uniform movement across the width of the microfluidic channel.
A schematic of the experimental set up can be seen below (figure 1)
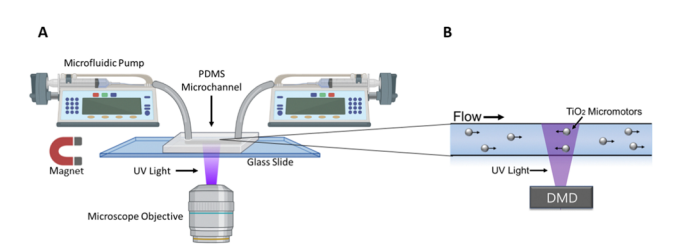
Figure 1:Adapted from Wu et al., (2023); (A) A microfluidic device is positioned on the stage of an inverted microscope. A microfluidic pump fills the channels of the device with micromotors in hydrogen peroxide solution. Their activity can then be observed through the objective of the microscope. (B) The Mightex Polygon DMD Pattern Illumınator is then used to project UV patterned stimulation to the field of view, resulting in controlled movement of the micromotors within the microfluidic device.
Methods
Application of UV light by the Polygon is able to generate movement in the micromotors by initializing a self-electrophoretic mechanism, such that a flow of charge is generated on the surface of the micromotor relative to the surrounding medium. This in turn results in an anisotropic electric charge that produces motion. When the patterned light from the Polygon is not present, the micromotors move passively with the fluid flow. When only a portion of the field of view and channel containing the micromotors is illuminated by the Polygon, particles in that region begin to move against the fluid flow, reaching dynamic equilibrium (active propulsion upstream balanced with passive flow downstream) at the edge of the patterned illumination.
Over time, the passive flow of micromotors into the illuminated region allows a growing cluster of micromotors to collect at the dynamic equilibrium point, creating a micromotor swarm. The result of this process is seen in Figure 2 and Video 1 below.
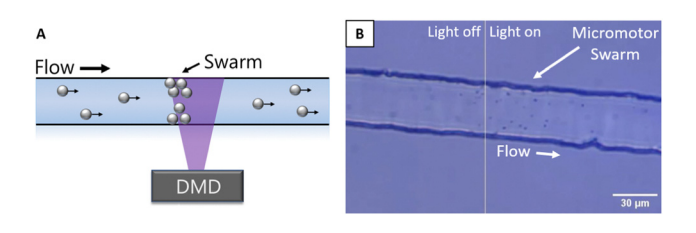
Figure 2: Adapted from Wu et al., (2023) (A) Schematic of the swarm creation process, using patterned illumination from the Polygon DMD to cluster micromotors at upstream edge in dynamic equilibrium. (B) Image of micromotor swarm at edge of patterned illumination region.
After micromotor swarm generation, Wu and colleagues were able to control the position and movement of the swarm by changing the illuminated portion of the field of view to target a different region of interest along the microfluidic channel. The swarm followed the change of conditions, moving to the new edge of illumination. This process can be observed in Figure 3 and Video 2 below. 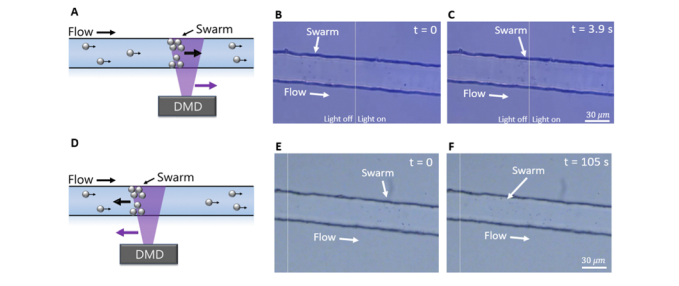
Figure 3: Adapted from Wu et al., (2023); (A) Schematic of formation of micromotor swarm and movement of patterned illumination to the right, (B) Image of swarm generated and location at time 0s after the patterned light has been moved to the right, (C ) Image of swarm 3.9s after movement of patterned light was moved, swarm has now relocated to the upstream bounding edge of the light. (D) Schematic of formation of micromotor swarm and movement of patterned illumination to the left, B) Image of swarm generated and location at time 0s after the patterned light has been moved to the left, (C ) Image of swarm 105s after movement of patterned light was moved, swarm has now relocated against fluid flow to the upstream bounding edge of the light.
Findings and Conclusion
In conclusion, Wu and colleagues have demonstrated the properties of light-inducible micromotor swarms at low hydrogen peroxide concentrations using the Mightex Polygon DMD. This work established the development of a micromotor swarm using dynamic equilibrium and patterned UV light. Furthermore, Wu and colleagues were then able to modify the patterned illumination location within a microfluidic channel, forcing active movement of the micromotors against the fluidic flow. This work has significant implications for development of biological assays using micromotors and microfluidic devices. In particular, this approach opens the door to versatile ‘Lab-on-a-chip’ tools, cell sorting, and drug delivery applications. We are thrilled that the Mightex Polygon is used for such ground-breaking research and look forward to reading exciting work from Wu and colleagues soon!
Catherine Thomas, PhD Senior Liaison and Development Scientist at Mightex
To read the full publication, please click here.



